Week LXXVI: Approved vax and new gov takes on Delta
ALBANY COUNTY — Long-held anticipation was answered this week as on Monday, the Food and and Drug Administration approved the first COVID-19 vaccine, Pfizer-BioNTech, and on Tuesday New York’s new governor, Kathy Hochul, said fighting the Delta variant of COVID-19 is her top priority.
“None of us want to see a rerun of last year’s horrors with COVID-19. Therefore we will take proactive steps to prevent that from happening,” Hochul said at her first press conference, on Tuesday afternoon.
Since the state of emergency ended in June, former Governor Andrew Cuomo had said it was up to individual municipalities to come up with guidance for COVID-19; few did.
At the same time, even as the Centers for Disease Control and Prevention labeled New York State as having a high rate of transmission, the state’s health commissioner, Howard Zucker, said it was up to individual school districts to come up with their own rules.
This put local school boards in the hot seat as parents opposed to mask-wearing made their views known.
Hochul took back the state’s leadership role on the issue.
Her first priority, she said on Tuesday, is, “We get children back to school and protect the environment so they can learn, and everyone is safe.”
This means requiring “vaccinations for all school personnel with an option to test out weekly — at least for now,” said Hochul.
New York is launching a Back to School COVID-19 testing program to make testing for students and staff widely available and convenient, she said.
“I am also immediately directing the Department of Health to institute universal masking for anyone entering our schools,” said Hochul.
Later this week, she said, she will announce a series of school-related policies “that will be concise and consistent, giving the school districts what they have been asking for.”
Her second priority, Hochul said, is to increase vaccination rates for New Yorkers.
“Much progress has been made, but too many are not yet vaccinated, putting themselves and their communities at risk,” she said. “With the FDA’s full approval of the Pfizer vaccine yesterday, New Yorkers can expect new vaccine requirements. More on that soon.”
Her third priority, the governor said, is to prepare for booster shots and make sure they are available and are distributed quickly and reliably.
“When I consulted with Dr. Fauci last week, we discussed the urgent need to ensure vaccinated individuals receive a booster dose at eight months,” Hochul said. “I am prepared to do whatever is necessary, including reopening mass vax sites so that a booster is available to all New Yorkers who meet that timetable.”
Hochul’s initiatives were embraced immediately in statements by Albany County’s executive, by the state’s largest teachers’ union, and by Zucker.
“A universal mask requirement for anyone entering our schools will give parents the peace of mind they deserve as they send their mostly unvaccinated children back to classrooms,” said Albany County Executive Daniel McCoy, “and it will help to avoid the confusion of fragmented rules in different school districts.
“Requiring vaccinations for school personnel with the option of testing out will be an added layer of protection, it will ensure in-person learning continues and it will help us reach herd immunity. Albany County is prepared to bolster the state’s testing efforts with the $6.6 million we’ve already been allotted from the federal government.”
“Since early July, COVID-19 cases in New York have risen 10-fold and 95 percent of sequenced positive cases were confirmed to be Delta variant,” said Zucker. “Based on incidence and prevalence, our findings demonstrate the necessity of layered prevention strategies, including this mask requirement. While a simple measure of prevention, requiring masks now is crucial for protecting the health of our children and ensuring we can get our students back in their schools this fall.”
New York State United Teachers President Andy Pallotta said, “We support universal mask-wearing as part of a layered mitigation strategy that also includes robust COVID testing, contract tracing, proper ventilation, and other strategies recommended by public-health experts. We also support the governor’s move to require regular COVID testing for school staff who are not yet vaccinated.”
FDA approval
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” said Acting FDA Commissioner Janet Woodcock, in a release on Monday announcing the approval.
The FDA approval opens the door for vaccination requirements both by governments and private entities. The state’s university system immediately required vaccination as did a number of businesses.
The Pfizer-BioNTech COVID-19 Vaccine was the first available, starting Dec. 11, 2020, under emergency use authorization from the FDA.
This emergency approval was based on safety and effectiveness data from a randomized, controlled, blinded ongoing clinical trial of thousands of individuals.
On May 10, 2021, the authorization was expanded to include people 12 through 15 years old.
Emergency use authorizations can be used by the FDA during public health emergencies to provide access to medical products that may be effective in preventing, diagnosing, or treating a disease, the FDA explained in the release, provided that the FDA determines that the known and potential benefits of a product outweigh the known and potential risks.
“For all vaccines, the FDA evaluates data and information included in the manufacturer’s submission of a biologics license application,” the FDA said.
The Pfizer-BioNTech vaccine will now be marketed as Comirnaty (koe-mir´-na-tee), for the prevention of COVID-19 disease in people 16 and older.
The vaccine also continues to be available under emergency use authorization, including for youth 12 through 15 years of age and for the administration of a third dose in certain immunocompromised individuals.
Comirnaty contains messenger RNA, a kind of genetic material. “The mRNA is used by the body to make a mimic of one of the proteins in the virus that causes COVID-19,” the FDA explained. “The result of a person receiving this vaccine is that their immune system will ultimately react defensively to the virus that causes COVID-19.
“The mRNA in Comirnaty is only present in the body for a short time and is not incorporated into — nor does it alter — an individual’s genetic material.” Comirnaty has the same formulation as the emergency use authorization vaccine and is administered as a series of two doses, three weeks apart.
In the FDA’s review for approval, the agency analyzed effectiveness data from approximately 20,000 vaccine and 20,000 placebo recipients ages 16 and older who did not have evidence of the COVID-19 virus infection within a week of receiving the second dose.
Based on results from the clinical trial, the vaccine was 91 percent effective in preventing COVID-19 disease, the FDA reports.
More than half of the clinical trial participants were followed for safety outcomes for at least four months after the second dose. Overall, approximately 12,000 recipients have been followed for at least six months.
The most commonly reported side effects were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever. “The vaccine is effective in preventing COVID-19 and potentially serious outcomes including hospitalization and death,” the FDA says.
“Additionally, the FDA conducted a rigorous evaluation of the post-authorization safety surveillance data pertaining to myocarditis and pericarditis following administration of the Pfizer-BioNTech COVID-19 Vaccine and has determined that the data demonstrate increased risks, particularly within the seven days following the second dose,” the FDA says.
Myocarditis is inflammation of the heart while pericarditis is inflammation of tissue surrounding the heart.
The risk is highest in males 12 through 17 years of age. “Available data from short-term follow-up suggest that most individuals have had resolution of symptoms,” the FDA says. “However, some individuals required intensive care support. Information is not yet available about potential long-term health outcomes.”
“We evaluated scientific data and information included in hundreds of thousands of pages, conducted our own analyses of Comirnaty’s safety and effectiveness, and performed a detailed assessment of the manufacturing processes, including inspections of the manufacturing facilities ...,” said Peter Marks, director of FDA’s Center for Biologics Evaluation and Research, in the release. “The public and medical community can be confident that although we approved this vaccine expeditiously, it was fully in keeping with our existing high standards for vaccines in the U.S.”
Expanded eligibility for small-business grants
On Wednesday, Hochul announced changes to the state’s $800 million COVID-19 Pandemic Small Business Recovery Grant Program that will enable more small businesses to apply for funding.
Starting on Aug. 25, businesses with revenues up to $2.5 million can apply for grants, up from the previous threshold of $500,000.
Additionally, the limitation for businesses that received Federal Paycheck Protection Program loans has been increased from $100,000 to $250,000.
“Supporting the small businesses across our state that got hit hard by the pandemic is a top priority for my administration,” Hochul said in a statement. “We simply cannot have a full economic recovery if the small business community continues struggling to survive.”
Launched in June, the program initially focused on small and micro-businesses across the state, which were largely left out of federal business recovery initiatives. Ao far, according to the governor’s office, more than $48 million has been awarded to over 2,380 small and micro-businesses in all 10 regions of the state.
ESD and Lendistry, the minority-led Community Development Financial Institution that was selected to administer the program, will continue to accept and review applications. All current applicants — those who have not finished their applications, have not uploaded documents, or have incomplete documentation — are encouraged to finalize their applications as soon as possible
Previously ineligible small businesses may start applying on Aug. 15, and those applications will start being processed on Wednesday, Sept. 8.
Newest numbers
Albany County’s 76th week of dealing with the pandemic brought another death. On Friday, Aug. 20, McCoy reported a woman in her eighties had died, bringing the county’s COVID-19 death toll to 389.
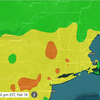
As of Wednesday evening, Albany County continues to have a high rate of transmission, the worst of four ratings, according to the Centers for Disease Control and Prevention. Only two counties in New York — Clinton and Yates — have a moderate rate of transmission.
The rest of New York’s counties have either substantial or high transmission rates, meaning masks should be worn in public indoors regardless of vaccination status.
Except for the state of Maine, the entire nation is red on the CDC map, meaning a high rate of transmission. Maine’s seven-day case rate is 90.9 per 100,000 of population, meaning its transmission rate is substantial.
New York’s high rate is 160.4 per 100,000. By contrast, Florida has a rate of 704.1 per 100,000 of population.
The United States as a whole is at 299.5 cases per 100,000 population.
On Tuesday, for just the fourth time, McCoy released numbers on COVID-19 cases in patients who are vaccinated: Among the 19 county residents who are currently hospitalized with the virus, he said, eight are fully vaccinated and 11 are not.
For the new COVID infections identified between Aug. 15 and 21, McCoy reported, 187 were fully vaccinated, 211 had not received a single shot, and for 25 individuals, the vaccination status was unknown. Vaccine status is self-reported by the case and provided without regard to the timing of vaccine administration and onset of illness.
Among the state’s 10 regions, as of Tuesday, as a seven-day average, three regions have infection rates over 4 percent: the North Country at 4.40 percent, Central New York at 4.18 percent, and the Capital Region at 4.16 percent.
Statewide, the infection rate is 3.13 percent. New York City is the region with the lowest rate at 2.52 percent.
Albany County, according to the state’s dashboard, as of Tuesday, as a seven-day average, has an infection rate of 4.0 percent.
On Wednesday morning, McCoy, in his daily release, reported 80 new cases of COVID-19, in keeping with high numbers all week long.
There are now 397 active cases in the county, up from 384 since Tuesday. The number of Albany County residents under quarantine increased to 639 from 606.
There were two new hospitalizations since Tuesday, and 19 county residents are now hospitalized with the virus — unchanged from Tuesday. There are still three patients in intensive-care units.
According to the state’s vaccine tracker, 68.8 percent of Albany County’s 307,117 residents have received at least one dose of vaccine as have 79.6 percent of residents 18 or older.
Statewide, 66.4 percent of New Yorkers have received at least one dose as have 78.8 percent of New Yorkers 18 or older. At the same time, 59.3 percent of New Yorkers are fully vaccinated as are 70.8 percent of New Yorkers 18 and older.