FDA approves Pfizer vaccine
ALBANY COUNTY — On Monday, the Food and Drug Administration approved the first COVID-19 vaccine, Pfizer-BioNTech.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” said Acting FDA Commissioner Janet Woodcock, in a release announcing the approval.
The FDA approval opens the door for vaccination requirements both by governments and private entities.
Monday was also Governor Andrew Cuomo’s last day in office. He had announced his resignation two weeks ago after a report from Attorney General Letitia James itemized sexual-harassment complaints from 11 women and the state legislature was gearing up for impeachment.
Bloodied but unbowed, Cuomo on Monday maintained, “The attorney general’s report was designed to be a political firecracker on an explosive topic. And it worked. There was a political and media stampede. But the truth will out in time — of that I am confident.”
His noontime address sounded rather like a campaign speech as he outlined his accomplishments over his decade as governor and his plans for the future of New York.
On COVID-19, Cuomo said, “We must focus on the immediate threat, which is the Delta variant.”
He went over New York’s success in going “from the highest infection rate in the nation to the lowest,” and gave advice for the future.
“School opening is approaching. Teachers must be vaccinated for their protection and for our children’s protection,” he said. “Masks must be required in high-risk areas and private businesses must mandate proof of vaccination for large gatherings.
“Now, this simply will not happen without a state law mandating that it happen. Local politics are too intense. Private businesses cannot and will not enforce the law. Local police must be mandated to do that, but we must take these actions.
“Let us remember political procrastination is COVID collaboration. We know the choice is between the politically contentious or the medically infectious. You decide which is worse.”
Late on Monday, at 5:30 p.m., Cuomo released a statement, calling for mandatory vaccination.
“This morning's announcement that the federal Food and Drug Administration has fully approved Pfizer’s COVID-19 vaccination eliminates any doubt in the science and efficacy of COVID vaccines,” he said. “In the wake of this decision, every single employer in New York State should require all eligible employees to get vaccinated, just like New York State already requires of all eligible government and health-care employees.
“It’s past time for pleading and cajoling — the vaccines are safe, effective, free, and readily available. The only way we’ll beat COVID once and for all is by getting every single eligible New Yorker vaccinated. No more excuses — let’s get it done, New York.”
Also on Monday, New York State United Teachers — a union with 600,000 members — and the New York State American Academy of Pediatrics launched a television and digital advertising campaign to highlight the importance of ensuring all students return to in-person instruction in the fall.
The ad focuses on the benefits of in-person learning — including hands-on instruction, social interaction, and extracurricular activities — with health protocols in place and agreement among educators and public-health experts that in-person learning can be done safely.
FDA approval
The Pfizer-BioNTech COVID-19 Vaccine was the first available, starting Dec. 11, 2020, under emergency use authorization from the FDA.
This emergency approval was based on safety and effectiveness data from a randomized, controlled, blinded ongoing clinical trial of thousands of individuals.
On May 10, 2021, the authorization was expanded to include people 12 through 15 years old.
Emergency use authorizations can be used by the FDA during public health emergencies to provide access to medical products that may be effective in preventing, diagnosing, or treating a disease, the FDA explained in the release, provided that the FDA determines that the known and potential benefits of a product outweigh the known and potential risks.
“For all vaccines, the FDA evaluates data and information included in the manufacturer’s submission of a biologics license application,” the FDA said.
The Pfizer-BioNTech vaccine will now be marketed as Comirnaty (koe-mir´-na-tee), for the prevention of COVID-19 disease in people 16 and older.
The vaccine also continues to be available under emergency use authorization, including for youth 12 through 15 years of age and for the administration of a third dose in certain immunocompromised individuals.
Comirnaty contains messenger RNA, a kind of genetic material. “The mRNA is used by the body to make a mimic of one of the proteins in the virus that causes COVID-19,” the FDA explained. “The result of a person receiving this vaccine is that their immune system will ultimately react defensively to the virus that causes COVID-19.
“The mRNA in Comirnaty is only present in the body for a short time and is not incorporated into — nor does it alter — an individual’s genetic material.” Comirnaty has the same formulation as the emergency use authorization vaccine and is administered as a series of two doses, three weeks apart.
In the FDA’s review for approval, the agency analyzed effectiveness data from approximately 20,000 vaccine and 20,000 placebo recipients ages 16 and older who did not have evidence of the COVID-19 virus infection within a week of receiving the second dose.
Based on results from the clinical trial, the vaccine was 91 percent effective in preventing COVID-19 disease, the FDA reports.
More than half of the clinical trial participants were followed for safety outcomes for at least four months after the second dose. Overall, approximately 12,000 recipients have been followed for at least six months.
The most commonly reported side effects were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever. “The vaccine is effective in preventing COVID-19 and potentially serious outcomes including hospitalization and death,” the FDA says.
“Additionally, the FDA conducted a rigorous evaluation of the post-authorization safety surveillance data pertaining to myocarditis and pericarditis following administration of the Pfizer-BioNTech COVID-19 Vaccine and has determined that the data demonstrate increased risks, particularly within the seven days following the second dose,” the FDA says.
Myocarditis is inflammation of the heart while pericarditis is inflammation of tissue surrounding the heart.
The risk is highest in males 12 through 17 years of age. “Available data from short-term follow-up suggest that most individuals have had resolution of symptoms,” the FDA says. “However, some individuals required intensive care support. Information is not yet available about potential long-term health outcomes.”
“We evaluated scientific data and information included in hundreds of thousands of pages, conducted our own analyses of Comirnaty’s safety and effectiveness, and performed a detailed assessment of the manufacturing processes, including inspections of the manufacturing facilities ...,” said Peter Marks, director of FDA’s Center for Biologics Evaluation and Research, in the release. “The public and medical community can be confident that although we approved this vaccine expeditiously, it was fully in keeping with our existing high standards for vaccines in the U.S.”
Newest numbers
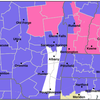
As of Monday evening, Albany County continues to have a high rate of transmission of COVID-19, according to the Centers for Disease Control and Prevention. Only two counties in New York — Clinton and Allegany — have a moderate rate of transmission.
The rest of New York’s counties have either substantial or high transmission rates, meaning masks should be worn in public indoors regardless of vaccination status.
On Monday morning, Albany County Executive Daniel McCoy reported 52 new cases of COVID-19, fewer than the 66 he reported on Monday, the 90 on Saturday, and the 78 cases he reported on Friday.
Also on Friday, McCoy reported the death of a woman in her eighties, bringing the county’s COVID-19 death toll to 389.
There are now 393 active cases in the county, down from 406 on Sunday. The number of county residents under quarantine decreased to 626 from 654.
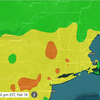
The infection rate in Albany County, as of Sunday as a seven-day average, was 3.9 percent, according to the state’s dashboard. Statewide, the infection rate was 3.2 percent.
The Capital Region, as of Sunday, as a seven-day rolling average, had the third worst infarction rate of the state’s 10 regions, at 4.2 percent. The worst was Central New York at 4.61 percent followed by the North Country at 4.25 percent.
Statewide, the infection rate was 3.16 percent. New York City had the lowest rate at 2.55 percent.
Albany County had one new hospitalization since Monday, and 20 county residents remain hospitalized with the virus. There are still three patients in intensive-care units, unchanged from Monday.
“Albany County continues to make progress in our vaccination efforts, as we inch closer and closer to an 80-percent first-dose vaccination rate for our 18-plus population …,” said McCoy in a statement. “This further supports what countless scientific studies have already shown — getting the shot is safe and effective. I hope that this will show even more people who are still on the fence about getting vaccinated that it is the right decision to make.”
According to the state’s vaccine tracker, 68.6 percent of Albany county’s 307,117 residents have received at least one shot as have 79.4 percent of county residents 18 and older.
Statewide, 66.2 percent of New Yorkers have received at least one shot and 59.1 percent are fully vaccinated. At the same time, among New Yorkers 18 and older, 78.6 have received at least one shot and 70.7 have completed a vaccine series.