Week CXVII: County COVID cases continue to decline, hope on the horizon for under-5 vax
ALBANY COUNTY — This week, for the first time in two months, Albany County had a week without a COVID-related death. At the same time, the infection rate and the cases per 100,000 of population continues to decline.
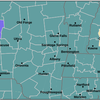
Still, Albany County is labeled by the Centers for Disease Control and Prevention as having a “high” community level of COVID-19. This means masks should be worn indoors in public.
Last week, the CDC map showed most of the counties in the center of the state — which had been the epicenter for the surge of COVID caused by Omicron subvariants — as having “medium” community levels. This week, most of those counties are colored green, signifying a “low” level.
But counties on the eastern edge of the state, like Albany County, continue to have high levels.
Also this week, The Food and Drug Adminstration’s advisory committee met to consider authorizing a fourth vaccine against COVID, made by Novavax. And the FDA is expected to make a decision shortly on allowing Pfizer-BioNTech and Moderna’s vaccines to be used for children younger than 5.
Long-term care facilities continue to feel the effects of staffing shortages, and New York State continues to offer free webinars to help workers who believe they contracted COVID-19 on the job get benefits.
Albany County
“Albany County’s daily average of new COVID infections as well as the number of cases per 100,000 continue to decline,” said County Executive Daniel McCoy in his Tuesday COVID release, “and the last time I had to report a new COVID-related death was back on May 27. All of this is good news, and I’m hopeful that our numbers will continue to move in the right direction through the summer months.
“With that being said, we should continue to stay vigilant. We still need more people to get vaccinated and get their booster shots in order to prevent serious illnesses, and everyone should continue to test and stay home if they’re feeling sick to prevent the spread of the virus.”
Albany County still has a quarter of its population that is not fully vaccinated. Among the eligible population, 63.8 percent of county residents have now received the booster shot, McCoy reported on Tuesday.
The rate of COVID cases per 100,000 of population is continuing the decrease it started on May 19. McCoy reported in his Tuesday COVID release that Albany County has 25.4 cases per 100,000 as a seven-day everage.
This compares with 38.2 last week, 49.6 two weeks ago, 51.2 three weeks ago, 54.2 cases per 100,000 four weeks ago, 43.7 five weeks ago, 37.7 six weeks ago, 28.3 cases seven weeks ago, 21.1 cases eight weeks ago, and 11.0 cases per 100,000 nine weeks ago.
The state’s count of cases per 100,000 of population, as a seven-day average, peaked at 51.0 on May 11. It is now down to 29.89, a dramatic drop from 41.41 last week.
Long Island still has the highest rate, at 38.61, a decrease from 45.7 last week and 62.72 the week before. Central New York still has the lowest rate at 10.48, a big drop from 16.30 last week, down from 22.44 cases per 100,000 of population the week before.
The less reliable infection rate — the percentage of positive test results — is now at 8.6 percent for Albany County as a seven-day average.
This is down from 11.6 percent last week, 13.1 two weeks ago, and 13.3 percent three weeks ago after a steady climb up: 12.2 percent four weeks ago, 10.0 percent five weeks ago, 13.5 percent six weeks ago, 9.1 percent seven weeks ago, 7.5 percent eight weeks ago, 3.5 percent nine weeks ago, and 2.6 percent 10 weeks ago.
Statewide, as a seven-day average, the infection rate is 5.95 percent, down from 6.82 percent last week, and 8.04 percent the week before. Central New York has the lowest rate at 4.04 percent while Long Island has the highest rate at 8.94 percent.
Hospitalizations typically lag behind infection rates.
Forty-one Albany County residents are currently hospitalized with COVID-19, with four of them in intensive-care units.
This compares with 48 hospitalized last week, 43 hospitalized two weeks ago, 42 three weeks ago, 51 four weeks ago, 34 five weeks ago, 31 six weeks ago, 30 county residents seven weeks ago, 21 county residents hospitalized eight weeks ago, and 13 hospitalized with the virus nine weeks ago.
So it appears that, after a steady increase for a month and a half, hospitalizations are still leveling off. Also, the governor’s office reports that 38.3 percent of people with COVID-19 hospitalized in the Capital Region were not admitted because of the virus.
Vaccines
On Tuesday, the FDA’s advisory committee considered a request made on Feb. 1 from Novavax for emergency use authorization of its vaccine for people 18 and older.
The vaccine is a “Recombinant Spike Protein Nanoparticle Vaccine,” according to the briefing document, and would consist of two injections.
The Novavax vaccine, which is different from the messenger RNA vaccines made by Moderna and Pfizer-BioNtech that have been in use for over a year, is composed of proteins called nanoparticles from the surface of the coronavirus.
Martha Dawson, the president of the National Black Nurses Association, told the FDA committee that some patients would feel more comfortable using protein-based vaccines, a technology that has been used for decades, The New York Times reported.
The Novavax clinical trial took place before the Delta and Omicron variants were predominant and showed a 90.4 percent efficacy of preventing mild, moderate, or severe infections with the earlier variants.
In December 2021, the World Health Organization issued an emergency use listing for the Novavax vaccine.
“Even with new variants emerging, vaccines remain one of the most effective tools to protect people against serious illness and death from SARS-COV-2,” said Dr. Mariângela Simão, WHO Assistant-Director General for Access to Medicines and Health Products, in the WHO announcement in December. “This listing aims to increase access particularly in lower-income countries, 41 of which have still not been able to vaccinate 10% of their populations, while 98 countries have not reached 40%.”
Hope builds that the only Americans not eligible for vaccination against COVID-19 — the very youngest — may soon be eligible.
Pfizer and BioNTech said in a release in May that three doses of their COVID vaccine in children aged 6 months up to 5 years old “was found to elicit a strong immune response, with a favorable safety profile.”
Vaccine efficacy was 80.3 percent., the companies said.
In February 2022, the companies had started a rolling submission for emergency use authorization of their COVID-19 vaccine in children 6 months to under 5 years of age, following a request by the FDA. At that time, the release notes, a two-dose series was determined to be well-tolerated in this age group.
Two doses of vaccine, however, proved not to be effective in preventing infection with the Omicron variant so the companies turned to testing a three-dose regimen.
On May 23, the FDA revised the dates of the upcoming Vaccines and Related Biological Products Advisory Committee meetings because of new data and expected submissions of emergency use authorization requests.
On June 14, the FDA and its advisory committee of external experts are to discuss Moderna’s emergency-use request for children 6 through 17 years old.
Then, the next day, June 15, Moderna’s emergency-use request for 6 months through 5 years of age will be considered along with the Pfizer-BioNTech emergency-use request for 6 months through 4 years of age.
The original schedule also included a committee meeting to discuss whether the SARS-CoV-2 strain composition of COVID-19 vaccines should be modified, and if so, which strain or strains should be selected for the fall of 2022.
Long-term care
On Monday and Tuesday, long-term care professionals met with members of Congress to push legislation they feel would address the workforce shortage.
“We all agree that nursing homes need to hire more caregivers — the question is how. Unfunded staffing mandates would only make the crisis worse. Congress must invest in our long term care workforce and protect access to care for millions of seniors,” said Mark Parkinson, president and chief executive officer of the American Health Care Association, in a release from the association, which represents more than 14,000 nursing homes and other long-term care facilities across the country.
The release cited a survey of nursing home providers across the United States that found 87 percent are currently facing moderate to high staffing shortages while 98 percent are experiencing difficulty hiring staff.
“Without enough staff, nursing homes cannot care for as many residents, and the already underfunded health care sector struggles to recover from the COVID-19 pandemic,” the release said.
The survey also found nearly six out of 10 nursing home providers operating at a loss, and 73 percent are concerned about having to close their facilities over staffing woes. Providers estimate that their costs have increased by an average of 41 percent since last year and 53 percent said they can’t sustain the current operating pace for more than one year.
Workers’ comp
The Workers’ Compensation Board is continuing its webinar series to help workers who believe they contracted COVID-19 on the job, especially those who have missed time from work or are suffering from ongoing or “long-haul” symptoms.
A session was held on Wednesday, June 8, and the next session is scheduled for Wednesday, July 13, from noon to 1 p.m.
Each session, according to a notice from the board, provides information on workers’ rights when it comes to filing a workers’ compensation claim and the cash or medical benefits they may be eligible to receive.
While the online sessions are targeted toward workers who have lost time from work, have ongoing medical problems, or fall into the category of “long haulers,” the information is relevant to anyone who believes they may have contracted COVID-19 due to an exposure at work.
Registration is not required. Online attendance is through this link: http://www.wcb.ny.gov/webinars/#covid-19.
At the end of each session, there is a time for questions to be answered.
In addition to the board’s dedicated COVID-19 web page, information and assistance with filing a claim for COVID-19 is available by calling the board at 877-632-4996.
Injured or ill workers are encouraged to let the board know if they face resistance or obstacles to getting information about the claims process or are discouraged from filing a claim. Such workers can send an email to for assistance.